To follow up on my post earlier this week, this is a deep dive into the VEXAS study published in January 2023. Arthritis, psoriasis, polymyalgia rheumatica, dermatomyositis, and sarcoidosis—these are all rheumatological signs, symptoms and diagnoses found in study participants with VEXAS. VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) is defined by disease-causing variants (mutations) on the gene UBA1. These are acquired variants that occur in those over 50 years old. UBA1 variants are x-linked, occurring more commonly in men.
Area of Investigation
VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic)
Study Title
Estimated Prevalence and Clinical Manifestations of UBA1 Variants Associated With VEXAS Syndrome in a Clinical Population
Results
The study included 163,096 participants. Out of those participants, 11 were found to have variants at disease-causing positions. 11 out of the 11 participants (9 male and 2 female), with potentially disease-causing variants, also had clinical manifestations of VEXAS syndrome. Interestingly, 5 out of the 11 participants, did not meet classification criteria for rheumatologic and/or hematologic diseases that had been previously associated with VEXAS syndrome. All 11 had anemia (low blood counts) with thrombocytopenia (low platelet count). One male was found to have a potentially disease-causing variant before the onset of his VEXAS-related signs and symptoms. The two female participants had disease with heterozygous variants. One new UBA1 variant was found in a symptomatic patient, with testing data supporting the potential of it being a disease-causing variant.
Prevalence
Disease-causing UBA1 variants were found in 1 in every 13,591 participants. The prevalence in older men was much higher, at 1 in 4,269 in men older than 50 years. Disease-causing variants were found in 1 in 26,238 women older than 50 years. There was a prevalence of 1 in 7,931 for all individuals older than 50 years, regardless of sex.
This research shows that VEXAS syndrome includes individuals older than 50 years without specific clinical rheumatologic diagnoses. It includes individuals “with a combination of hematologic, dermatologic, or pulmonary symptoms along with anemia, thrombocytopenia, and elevated inflammatory markers.” According to the authors, VEXAS syndrome lacks an ICD-10 code. This is the code physicians use for diagnosis and billing. The fact that this is absent means that VEXAS cannot be included officially in a diagnosis list, and there’s no mechanism for related testing to be covered by insurance. Considering this lack, participants were considered to have VEXAS if 2 organ systems, known to be associated with VEXAS, were affected, and the participants were anemic or had low platelets.
“At an occurrence of 1 in 13,591 across all age groups,” disease-causing UBA1 variants could be more common than the diagnoses that previously made up VEXAS syndrome, “including most vasculitides:”
Granulomatosis with polyangiitis has an approximate prevalence rate of 1 in 18,000
Polyarteritis nodosa has an approximate prevalence rate of 1 in 33,000.
The authors note the diseases that have similar prevalence rates
Behcet disease has an approximate prevalence rate of 1 in 10,000
Myelodysplastic syndrome has an approximate prevalence rate of 1 in 14,000.
Given these results, using signs and symptoms to identify VEXAS, was likely to be significantly inaccurate.
Despite the large number of cases reported to date, UBA1 is still not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels. Establishing the clinical spectrum of UBA1 variants using biobanks is a necessary step to better define disease criteria and testing recommendations.
The identification of disease-causing UBA1 variants is what defines VEXAS syndrome. The accurate identification of VEXAS can alter treatment, prognosis, and screening plans. The authors recommend broad UBA1 testing in patients with features consistent with what is known about VEXAS to date, including those with macrocytic anemia of unknown cause when it’s associated with elevated inflammatory markers.
Factors Associated with VEXAS
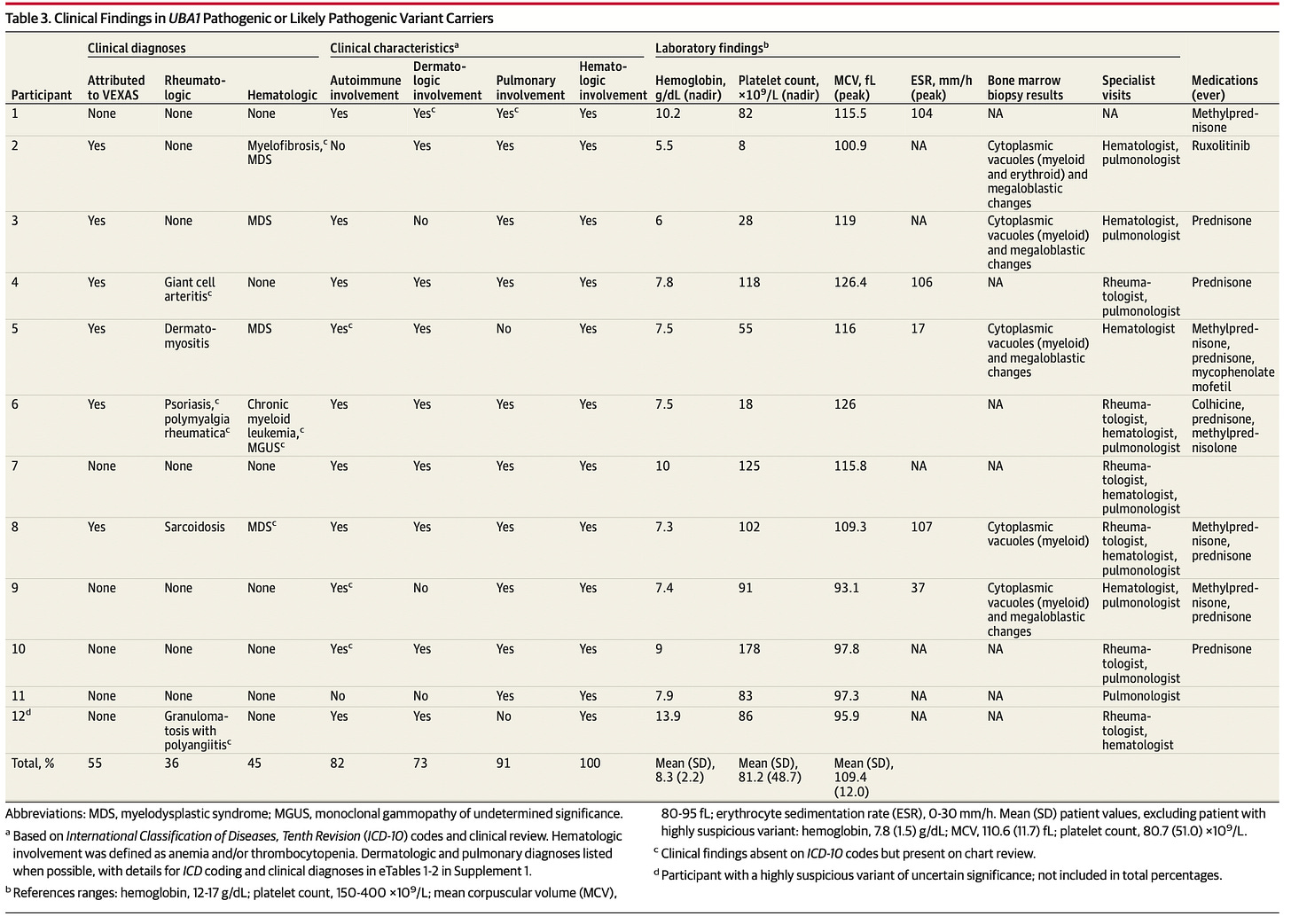
All 11 carriers of potentially disease-causing variants were older than 50 years at the onset of symptoms. This is consistent with previous reports on VEXAS. 7 out of 11 participants had arthritis and 4 were diagnosed with rheumatological diseases. Those rheumatological diseases included psoriasis, polymyalgia rheumatica, dermatomyositis, and sarcoidosis. Only 4 out of 11 participants had features consistent with what was previously considered consistent with VEXAS flares (fevers, inflammatory markers, steroid dependency). 11 out of 11 had anemia, 10 out of 11 had larger than normal red blood cells (macrocytosis), 5 out of 11 required transfusions. 10 out of 11 had low platelets, 4 out of 11 had Myelodysplastic syndrome, 1 out of 11 had monoclonal gammopathy of undetermined significance, and 1 out of 11 had chronic myelogenous leukemia. 8 out of 11 had skin involvement and 10 out of 11 had lung disease. Below is a chart of clinical findings associated with participants with disease-causing variants of UBA1, in case chart form is how you prefer your data.
Why it Matters
VEXAS is a prime example of how Whole Exome Sequencing, not diagnosis by the signs and symptoms associated with body system, is the key to unlocking accurate autoimmune disease diagnosis.
Study Type
Limitations
This study has several limitations. First, this study is based on a single-center regional cohort that may not be representative of other geographic locations and ancestries due to a population that is predominantly of European ancestry.
The chart below illustrates this point.
(For a critique of BMI as a health measure, the Weight and Healthcare substack is terrific.)
Second, this work is limited to clinical care received within a single health system and cannot account for missing data from clinical findings, treatment, or testing provided by an external clinician not captured in the Geisinger EHR available as part of the study. Third, UBA1 somatic variant detection on exome data may be limited due to sequencing depth and coverage, use of peripheral blood rather than bone marrow samples, and the possibility of novel and currently unrecognized pathogenic variants. Fourth, prevalence may be overestimated if highly suspicious variants are not validated as causal in other studies. Although clinical diagnoses were likely underrecorded because of diagnostic and physician coding challenges and cohort-specific approaches and characteristics, both in this study and those for other diagnoses, the reported UBA1 variant prevalence is also likely underestimated due to the requirements for sequence depth to detect low-level somatic variants. Additional cohort analyses will be critical to continue to evaluate and better understand the prevalence, penetrance, and expressivity of UBA1 variants in diverse populations.
For reference, I have ranked medical study types in order of least likely to be affected by hidden bias to most likely to be affected. Those studies that are most likely to be affected by hidden bias should be taken seriously, but not given the same weight as studies that are less likely to be affected by hidden bias. This study’s type appears in bold below.
Clinical Trial
Observational Study
Prospective
Retrospective
Cross-sectional
I will be on vacation next week. I plan to resume posting on a weekly basis the following week. I truly appreciate your readership.
References
Beck DB, Bodian DL, Shah V, et al. Estimated Prevalence and Clinical Manifestations of UBA1 Variants Associated With VEXAS Syndrome in a Clinical Population. JAMA.2023;329(4):318–324. doi:10.1001/jama.2022.24836