Scleroderma
| Systemic sclerosis | Localized scleroderma | Morphea | Eosinophilic fasciitis
Summary
Scleroderma is subdivided into two categories: Systemic sclerosis and Localized scleroderma. Localized scleroderma is also sometimes called Morphea, and is split into different sub-types. The sub-types of Localized scleroderma vary, depending on which categorical standard is being used. Eosinophilic fasciitis may be a severe form of Morphea. Expert opinion differs on how to define Scleroderma, in all its many forms.
Scleroderma means hard skin. It is an autoimmune related disease that causes abnormal growth of connective tissue. Connective tissue is the material inside your body that gives your tissues their shape and helps keep them strong. In scleroderma, the tissue gets hard or thick. It can cause swelling or pain in your muscles and joints. Symptoms of scleroderma include: calcium deposits in connective tissues, Raynaud’s phenomenon, a narrowing of blood vessels in the hands or feet, swelling of the esophagus, thick, tight skin on your fingers, and red spots on your hands and face. No one knows what causes scleroderma. It is more common in women. It can be mild or severe. Doctors diagnose scleroderma using your medical history, a physical exam, lab tests, and a skin biopsy. There is no cure, but various treatments can control symptoms and complications.
(2022, Autoimmune Association)
An overproduction of collagen protein which leads to the hardening and inflammation of connective tissues and skin. Scleroderma can be localized, meaning it only affects the skin, or it can be systemic, meaning it affects other parts of the body as well (such as the heart, lungs, kidneys, and digestive tract).
(2022, Global Autoimmune Institute)
Symptoms
Scleroderma's signs and symptoms vary from person to person, depending on which parts of the body are affected.
Skin-related signs and symptoms
Nearly everyone who has scleroderma experiences a hardening and tightening of the skin.
The first parts of the body to be affected are usually the fingers, hands, feet and face. In some people, the skin thickening can also involve the forearms, upper arms, chest, abdomen, lower legs and thighs. Early symptoms may include swelling and itchiness. Affected skin can become lighter or darker in color and may look shiny because of the tightness.
Some people also experience small red spots, called telangiectasia, on their hands and face. Calcium deposits can form under the skin, particularly at the fingertips, causing bumps that can be seen on X-rays.
Raynaud's phenomenon
Raynaud's phenomenon is common in scleroderma and occurs because of an inappropriate and exaggerated contraction of the small blood vessels in the fingers and toes in response to the cold or emotional distress. When this happens, the digits may turn white, blue or red, and feel painful or numb. Raynaud's phenomenon also can occur in people who don't have scleroderma.
Digestive problems
Scleroderma can affect any part of the digestive system, from the esophagus to the rectum. Depending on which parts of the digestive system are affected, signs and symptoms may include:
Heartburn
Difficulty swallowing
Bloating
Diarrhea
Constipation
Fecal incontinence
Heart and lung problems
When scleroderma affects the heart or lungs, it can cause shortness of breath, decreased exercise tolerance and dizziness. Scleroderma can cause scarring in the lung tissues that may result in increasing shortness of breath over time. There are medications that may help slow the progression of this lung damage.
Scleroderma can also cause the blood pressure to increase in the circulation that goes between the heart and the lungs. This is called pulmonary hypertension. In addition to shortness of breath, pulmonary hypertension can also cause excess fluid in the legs, feet and sometimes around the heart.
When scleroderma affects the heart, heartbeats can become irregular. Heart failure may also occur in some people.
(2022, Autoimmune Association)
Patches of skin that are hard and thick, rigid joints and joint pain, fatigue, heartburn, calcium deposits under the skin, weight loss, hair loss, red spots on the body, decreased blood flow to fingers, persistent cough, painful mucosal membranes (such as in the vaginal area), and gastrointestinal problems.
(2022, Global Autoimmune Institute)
Diagnostic Criteria
Systemic sclerosis
None. (Aggarwal et al, 2015).
Localized scleroderma/Morphea
Preliminary, unvalidated, diagnostic criteria for Localized scleroderma were proposed in 2018:
“Cases must satisfy all three of the following items:
Presence of sclerodermatous skin changes with circumscribed borders
Histopathological examination shows thickened and increased collagen fibers in the dermis
The following diseases can be excluded (however, this excludes cases in which the following diseases occur concurrently): systemic sclerosis, eosinophilic fasciitis, lichen sclerosus et atrophicus, keloid, (hypertrophic) scars and sclerosing panniculitis.”
(Asano et. al, 2018)
Prior to 2018, Localized scleroderma had a long history of being “classified” by its sub-types, without an overarching definition for Localized scleroderma itself. Here’s an accounting of the sub-types:
Tuffanelli and Winkelmann classification (1961)
Morphea
“characterized by circumscribed, sclerotic plaques with an ivory-colored center and surrounding violaceous halo.
Punctate morphea is considered to be a variant of morphea, in which there appear small plaque complexes”
Linear scleroderma
“appears in a linear, band-like distribution, and scleroderma en bondes is a synonym of linear scleroderma. Frontal or frontoparietal linear scleroderma (en coup de sabre) is characterized by atrophy and a furrow or depression that extends below the level of the surrounding skin”
Generalized morphea
“the most severe form of localized scleroderma, is characterized by widespread skin involvement with multiple indurated plaques, hyperpigmentation and frequent muscle atrophy”
Generalized morphea classification criteria proposed by Sato et al (1994)
“A case is classified as having generalized morphea if both the following criteria are satisfied:
Four or more skin lesions that measure 3 cm or more in diameter (irrespective of whether the skin lesions are patchy or linear)
The skin lesions are distributed in two or more sites of the seven regions of the body (head and neck, left and right upper limbs, trunk front and back, and left and right lower limbs).
If the above criteria are not satisfied simultaneously, the condition is classified as morphea or linear scleroderma based on the morphological characteristics of the skin lesions”
Peterson et al. classification (1995)
Plaque morphea
Plaque morphea
Guttate morphea
Atrophoderma of Pasini and Pierini
Keloid morphea (nodular morphea)
(Lichen sclerosus et atrophicus)
Generalized morphea
Bullous morphea
Linear morphea
Linear morphea (linear scleroderma)
Morphea en coup de sabre
Progressive facial hemiatrophy
Deep morphea
Morphea profunda
Subcutaneous morphea
Eosinophilic fasciitis
Pansclerotic morphea of childhood
This criteria is rejected by Asano et. al “because it includes diseases for which a consensus had not been reached in terms of the spectrum of this condition (atrophoderma of Pasini and Pierini, lichen sclerosus et atrophicus and eosinophilic fasciitis), and this classification also does not have a proposal for which disease type a case should be classified if it satisfies more than one characteristic” (2018).
Padua Consensus classification (2004)
Circumscribed morphea
Superficial
Deep
Linear scleroderma
Trunk/limbs
Head
Generalized morphea
Pansclerotic morphea
Mixed morphea
“The Padua Consensus classification is dual, which means that the boundaries between individual disease types are somewhat blurred, but it is considered to be useful in clinical practice because by adding the histological criteria, it clearly categorizes the clinically important disease types, such as circumscribed morphea/deep variant and pansclerotic morphea. If we consider that the Padua Consensus classification is used as the global standard for classification of localized scleroderma disease types, we recommend classifying localized scleroderma into five different disease types: circumscribed morphea, linear scleroderma, generalized morphea, pansclerotic morphea and mixed morphea. The evidence level is low, but the recommendation level is set as 1D, based on the consensus of the committee that created this guideline.” (Asano et. al, 2018)
Eosinophilic fasciitis
Please see the Diagnosis Description for Eosinophilic fasciitis.
Study Classification Criteria
Systemic Sclerosis (SSc)
(van den Hoogen et. al, 2013)
ACR = American College of Rheumatology. EULAR = European League Against Rheumatism.
Localized scleroderma/Morphea
Variable.
Eosinophilic fasciitis
Variable. Please see the Diagnosis Description for Eosinophilic fasciitis for more information.
Diagnostic Tests
Systemic sclerosis
Nailfold capillaroscopy, a microscopic evaluation of the appearance of the small blood vessels, known as capillaries, found at the origin of the nailbed. In systemic sclerosis, capillaries in this area look abnormal, and probably contribute to Raynaud’s phenomenon in the fingers.
Lung function tests
anti-Scl-70 antibodies
antitopoisomerase-1 antibodies (ATA) “ATAs were strongly predictive for SSc {Systemic sclerosis} with a nine-fold probability of SSc occurrence in primary patients with RP {Raynaud’s Phenomenon}. The presence of both ATAs and scleroderma patterns of nailfold capillaroscopy may increase the prediction accuracy and susceptibility {refers to early recognition in patients with Raynaud’s phenomenon, who do not yet have symptoms more specific to Systemic sclerosis}.” (Yang et. al, 2020)
anti-centromere antibodies
anti-RNA polymerase III antibodies
Localized scleroderma/Morphea
visual exam (Papara et. al, 2023)
biopsy is not necessarily recommended “A skin biopsy for histopathological evaluation is usually reserved for atypical, doubtful cases. The biopsy has to be sufficiently deep, since some types of morphea affect the subcutis or underlying fascia and muscle. However, there are no specific histopathology features for morphea and routine histopathology can neither differentiate among the various types nor to distinguish it from SSc. Still, it can provide details regarding the disease state. Early inflammatory skin lesions show: (i) thick collagen bundles in the reticular dermis that run parallel to the skin surface, (ii) dense inflammatory infiltrates comprising lymphocytes, eosinophils, plasma cells and histiocytes between the collagen bundles, in the perivascular and periadnexal areas, (iii) normal or atrophic overlying epidermis. Later fibrotic skin lesions become less inflammatory, avascular with thickened blood vessel walls and narrow lumens, and collagen bundles get thick, compact, and highly eosinophilic with few or absent sweat glands. In addition, collagen may replace the underlying subcutaneous tissue.” (Papara et. al, 2023)
Complete Blood Count (CBC), includes types of white blood cells present in the blood (Papara et. al, 2023)
Complete Metabolic Panel (CMP) to test kidney and liver function to rule out Systemic sclerosis (Papara et. al, 2023)
Creatine kinase for suspected co-occurring myositis (Papara et. al, 2023)
Rheumatoid Factor for suspected co-occurring Rheumatoid arthritis (Papara et. al, 2023)
C-Reactive protein to check general inflammation
Anti-nuclear antibody test, elevated in up to 70% of patients (Papara et. al, 2023)
Ultrasound can accurately assess disease activity and damage (Papara et. al, 2023)
Eosinophilic fasciitis
Please see the Diagnosis Description for Eosinophilic fasciitis for more information.
Organized Autoimmunity
(Alternative Autoimmune Disease Classification: FIEM, MIEM or BIEM, or FEM, MEM or BEM)
sex predominance (is an autoimmune disease primarily found in genetic Females, Males, or equally in Both?)
Systemic sclerosis
Female (Peoples et. al, 2016)
Localized scleroderma/Morphea
Female (Papara et. al, 2023)
Eosinophilic fasciitis
Unknown (Mertens et. al, 2017)
One study of 34 participants with Eosinophilic fasciitis: 20 female (59%), 14 male (Lebeaux et. al, 2012).
One study of 63 participants with Eosinophilic fasciitis: 43 female (68%), 20 (32%) male (Wright et. al, 2016).
One study of 10 participants with Eosinophilic fasciitis: 6 female, 4 male (Tull et. al, 2018)
One study of 78 participants with Eosinophilic fasciitis: 47 female (60%), 31 male (40%) (Li et. al, 2020)
One study of 45 participants with Eosinophilic fasciitis: 35 male (77.8%), 10 female (Yang et. al, 2023)
One study of 89 participants with Eosinophilic fasciitis had a female to male ratio of 1:1 (Mango et. al, 2020)
Inherited and acquired gene variations that cause increased susceptibility
Human Leukocyte Antigen (HLA) Associations
Systemic sclerosis
Unclear (Yang et. al, 2020)
Localized scleroderma
Class I HLA B*37 (Jacobe et. al, 2014)
Generalized Morphea
HLA DRB1*15:01 (Jacobe et. al, 2014)
Linear Morphea
One study found no specific Class II HLA associations with this sub-type (Jacobe et. al, 2014)
Eosinophilic fasciitis
Not specifically studied in patients with Eosinophilic fasciitis.
Other Gene Variations (mutations)
Systemic sclerosis
(Yang et. al, 2020)
Localized scleroderma/Morphea
Under Investigation
Eosinophilic fasciitis
Unstudied, at this time.
Gene Triggering Environmental Exposures
Infections
Systemic sclerosis
COVID-19 (Chang et. al, 2023, found a significantly higher risk of Systemic sclerosis following COVID-19 infection in a study of 3,814,479 participants)
Localized scleroderma/Morphea
Under Investigation
Eosinophilic fasciitis
Borrelia burgdorferi, better known as the Lyme disease spirochete (Granter et. al, 1996). The case series cited includes four patients with spirochete identification, two with a definitive identification of Borrelia burgdorferi.
Drugs
Systemic sclerosis
Under Investigation
Localized scleroderma/Morphea
Under Investigation
Eosinophilic fasciitis
Check Point Inhibitors, a class of relatively new cancer treatment drugs. The following drugs in this class have been implicated in the development of Eosinophilic fasciitis:
Nivolumab (Le Tallec et. al, 2020 and Chan et. al, 2020 and Teboul et. al, 2022)
atezolizumab (Chan et. al, 2020)
pembrolizumab (Chan et. al, 2020 and Zampeli et. al, 2021)
Statins, a well-known class of drugs used to treat increased levels of cholesterol. This study used an international drug adverse reaction reporting database (Vigibase) to conduct their study. Due to its retrospective, observational design, I’m only listing the drugs with more than one adverse reaction reported.
Fluvastatin (Teboul et. al, 2022)
Atorvastatin (Teboul et. al, 2022)
Toxins
Systemic sclerosis
Under Investigation
Localized scleroderma/Morphea
Under Investigation
Eosinophilic fasciiitis
1980s outbreak of Eosinophilic fasciitis in Spain due to adulterated rapeseed oil (Mazilu et. al, 2023)
Stress
Systemic sclerosis
Needs to be assessed for each patient
Localized scleroderma/morphea
Needs to be assessed for each patient
Eosinophilic fasciitis
Strenuous exercise was associated with 46% and 28% of Eosinophilic fasciitis cases in two case series (Mertens et. al, 2017)
Needs to be assessed for each patient
Multiple interactive and destructive immune system pathologies
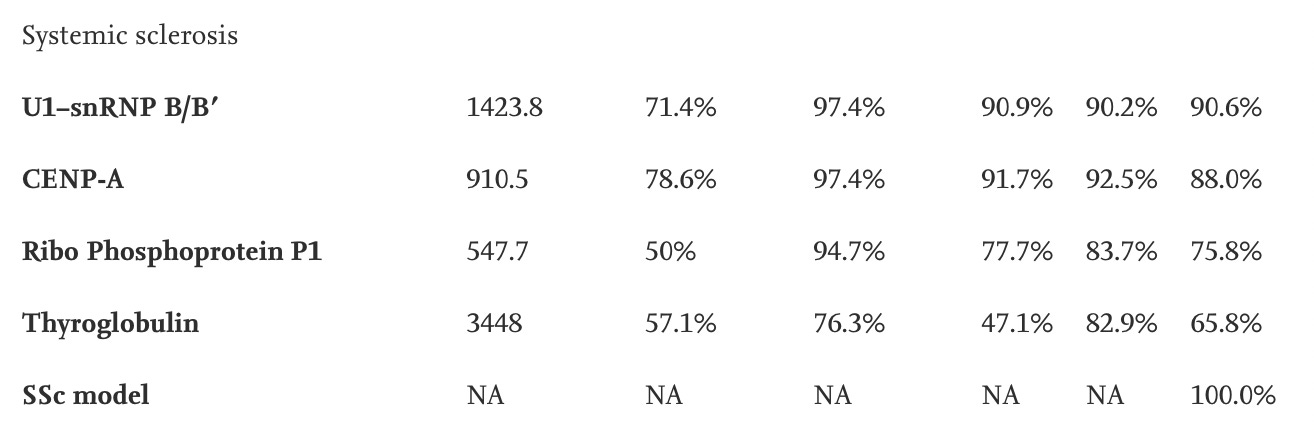
Systemic sclerosis
(2021, Rojas et. al)
PPV: positive predictive value (the probability that a patient with a positive (abnormal) test result actually has the disease). NPV: negative predictive value (the probability that a person with a negative (normal) test result is truly free of disease). (1999, NY State Department of Health). AUC: area under the curve.
Localized scleroderma/Morphea
Myelin basic protein autoantibodies are found in greater frequency, compared to systemic sclerosis (Zhu et. al, 2022)
antibodies against single-stranded DNA (ssDNA) have been associated with functional impairment in the linear subtype, but are present in low frequency, limiting clinical utility (Zhu et. al, 2022)
anti-histone antibodies have been associated with functional impairment in the linear subtype, but are present in low frequency, limiting clinical utility (Zhu et. al, 2022)
anti-human IgG/Rheumatoid factor (Zhu et. al, 2022)
anti-U1RNP (RNP = ribonucleoprotein) (Zhu et. al, 2022)
anti-MMP-1 (MMP = matrix metallopeptidase 1) (Zhu et. al, 2022)
Eosinophilic fasciitis
Moy et. al compared the tissue pathology of 12 participants with Localized scleroderma (morphea) to 8 participants with Eosinophilic fasciitis. They found that the T Helper 1 (Th1) and T Helper 2 (Th2) cells were significantly lower in Localized scleroderma compared to Eosinophilic fasciitis. T Helper 17 (Th17) cells were significantly higher in Eosinophilic fasciitis. This finding could help to differentiate between these conditions. (Moy et. al, 2017)
For more explanation on T Helper cells: “Effector Th1 cells produce proinflammatory cytokines such as INF-γ and lymphotoxin-α. These cytokines organize inflammatory centers and enhance cellular immune response; moreover, intracellular pathogens such as Mycobacteria and Salmonella spp, and other intravesicular agents are killed by IFN-γ through the activation of antimicrobial defenses. Th1 cytokine production is also characteristic of many organ-specific autoimmune diseases, including rheumatoid arthritis, insulin-dependent diabetes mellitus, experimental autoimmune encephalitis, and others. Effector Th2 cells, in contrast, produce a different profile of cytokines (IL-4, IL-5, IL-9, IL-10, IL-13, and so on) that together instruct B cells to proliferate and differentiate into antibody-secreting plasma cells, and potentiate the function of several cell types in antiparasite responses. As such, Th2 cells play an important role in providing protection against certain extracellular pathogens, such as bacteria and a variety of parasites, and are also involved in asthmatic reactions.” (Dong & Flavell, 2000)
Tissue-Type or Cell-Type Attacked
Systemic sclerosis
Small blood vessels, known as capillaries.
Skin
interstitial lung tissue
Localized scleroderma/Morphea
Skin tissue
fat tissue under the skin layers
fascia down to the bone.
Eosinophilic fasciitis
Please see the Diagnosis Description for Eosinophilic fasciitis for more information.
Treatment(s)
Systemic sclerosis
Hematopoietic stem cell transplantation in quickly deteriorating patients (Zhang et. al, 2023)
“Cyclophosphamide remains the first-line drug for therapy in systemic sclerosis complicated by pulmonary fibrosis” (Zhang et. al, 2023)
Localized scleroderma/Morphea
Corticosteroids (Papara et. al, 2023)
Methotrexate (Papara et. al, 2023)
Eosinophilic fasciitis
Please see the Diagnosis Description for Eosinophilic fasciitis for more information.
Managing Specialist(s)
Systemic sclerosis
Rheumatologist
Localized scleroderma/Morphea
Dermatologist
Pediatrician (In the past, Morphea has been considered a pediatric disease)
Pediatric dermatologist
Eosinophilic fasciitis
Dermatologist
Research Authors
Too numerous to list due to the many Scleroderma sub-types and sub-sub-types.
Research Institutions
Morphea in Adults and Children (MAC) registry. Registration is open. About: “Established in 2007 at the University of Texas Southwestern Medical Center, the MAC cohort is a prospective registry designed to better understand the demographic, clinical, and autoimmune features of morphea. Patients were enrolled from within the University of Texas Southwestern Medical Center system, which includes a county hospital, a faculty-based practice, and 2 dedicated pediatric care facilities. In addition, patients were recruited through referrals from pediatric and adult rheumatologists and dermatologists at the regional and national levels in an effort to enroll patients of varied disease severity, socioeconomic, and demographic backgrounds.” (Condie et. al, 2014)
The University of Texas Medical Branch at Galveston (UTMB), the University of Texas Health Science Center at Houston (UTHSC-H), and the University of Texas Health Science Center at San Antonio conduct the Scleroderma registry: Genetics versus Environment in Scleroderma Outcomes Study (GENISOS)
Average Time from Symptom Onset to Diagnosis
Under Investigation
Last Updated
September 22, 2023
References
Aggarwal, R., Ringold, S., Khanna, D., Neogi, T., Johnson, S.R., Miller, A., Brunner, H.I., Ogawa, R., Felson, D., Ogdie, A., Aletaha, D. and Feldman, B.M. (2015), Distinctions Between Diagnostic and Classification Criteria?. Arthritis Care & Research, 67: 891-897. https://doi.org/10.1002/acr.22583
Asano, Y., Fujimoto, M., Ishikawa, O., Sato, S., Jinnin, M., Takehara, K., Hasegawa, M., Yamamoto, T. and Ihn, H. (2018), Diagnostic criteria, severity classification and guidelines of localized scleroderma. J Dermatol, 45: 755-780. https://doi.org/10.1111/1346-8138.14161
Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine. 2023 Feb;56:101783. doi: 10.1016/j.eclinm.2022.101783. Epub 2023 Jan 10. PMID: 36643619; PMCID: PMC9830133.
Condie D, Grabell D, Jacobe H. Comparison of outcomes in adults with pediatric-onset morphea and those with adult-onset morphea: a cross-sectional study from the morphea in adults and children cohort. Arthritis Rheumatol. 2014 Dec;66(12):3496-504. doi: 10.1002/art.38853. PMID: 25156342; PMCID: PMC4245331.
Jacobe H, Ahn C, Arnett FC, Reveille JD. Major histocompatibility complex class I and class II alleles may confer susceptibility to or protection against morphea: findings from the Morphea in Adults and Children cohort. Arthritis Rheumatol. 2014 Nov;66(11):3170-7. doi: 10.1002/art.38814. Erratum in: Arthritis Rheumatol. 2015 Mar;67(3):751. PMID: 25223600; PMCID: PMC4211936.
Papara C, De Luca DA, Bieber K, Vorobyev A, Ludwig RJ. Morphea: The 2023 update. Front Med (Lausanne). 2023 Feb 13;10:1108623. doi: 10.3389/fmed.2023.1108623. PMID: 36860340; PMCID: PMC9969991.
Peoples C, Medsger TA Jr, Lucas M, Rosario BL, Feghali-Bostwick CA. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. J Scleroderma Relat Disord. 2016 May-Aug;1(2):177-240. doi: 10.5301/jsrd.5000209. Epub 2016 Jul 23. PMID: 29242839; PMCID: PMC5726425.
Rojas M, Ramírez-Santana C, Acosta-Ampudia Y, Monsalve DM, Rodriguez-Jimenez M, Zapata E, Naranjo-Pulido A, Suárez-Avellaneda A, Ríos-Serna LJ, Prieto C, Zambrano-Romero W, Valero MA, Rodríguez Y, Mantilla RD, Zhu C, Li QZ, Toro-Gutiérrez CE, Tobón GJ, Anaya JM. New insights into the taxonomy of autoimmune diseases based on polyautoimmunity. J Autoimmun. 2022 Jan;126:102780. doi: 10.1016/j.jaut.2021.102780. Epub 2021 Dec 16. PMID: 34923432.
Scleroderma. Autoimmune Association. (2022, February 10). Retrieved November 25, 2022, from https://autoimmune.org/disease-information/scleroderma/
Scleroderma. Global Autoimmune Institute. (2022). Retrieved November 25, 2022, from https://www.autoimmuneinstitute.org/scleroderma/
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr, Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Müller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Csuka ME, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013 Nov;65(11):2737-47. doi: 10.1002/art.38098. Epub 2013 Oct 3. PMID: 24122180; PMCID: PMC3930146.
Yang C, Tang S, Zhu D, Ding Y, Qiao J. Classical Disease-Specific Autoantibodies in Systemic Sclerosis: Clinical Features, Gene Susceptibility, and Disease Stratification. Front Med (Lausanne). 2020 Nov 19;7:587773. doi: 10.3389/fmed.2020.587773. PMID: 33330547; PMCID: PMC7710911.
Zhang Y, Zhu M, Xie L, Zhang H, Deng T. Identification and validation of key immune-related genes with promising diagnostic and predictive value in systemic sclerosis. Life Sci. 2023 Jan 1;312:121238. doi: 10.1016/j.lfs.2022.121238. Epub 2022 Nov 29. PMID: 36460097.
Zhu JL, Paniagua RT, Chen HW, Florez-Pollack S, Kunzler E, Teske N, Rainwater YB, Li QZ, Hosler GA, Li W, Ramirez DMO, Monson NL, Jacobe HT. Autoantigen microarrays reveal myelin basic protein autoantibodies in morphea. J Transl Med. 2022 Jan 24;20(1):41. doi: 10.1186/s12967-022-03246-5. PMID: 35073943; PMCID: PMC8785566.