Who coded what in the which, now?
HLA-DRB1 & Polymyalgia rheumatica
(Image Courtesy of: National Human Genome Research Institute)
HLA-DRB1 has been broadly associated with Polymyalgia rheumatica and other autoimmune diseases, particularly Rheumatoid arthritis. To learn more about Polymyalgia rheumatica, you can read its Diagnosis Description here. But what are HLAs specifically, and how are they relevant to autoimmune disease?
Some Background on HLAs
HLA is the acronym for Human Leukocyte Antigen. HLAs are self-antigens found on the human body’s cells. An antigen is any molecule that an antibody can react with. Circulating white blood cells (extracted with a standard blood draw) are used in a lab to analyze the presence of particular HLAs, which is why the term leukocyte (white blood cell) is used in the name. HLAs are not exclusive to white blood cells. When I think of antigens on human cells, I imagine the spherical portion of the medieval weapon, the Morningstar (pictured below).

If that iron sphere was a human cell, then its spikes are the antigens that an antibody uses to identify a cell it perceives as foreign, and either destroy that cell, or give its antigenic signature to another immune system component for destruction. It’s important to note that antigens can also be contained within cells, but cell surface antigens are easier to visualize.
HLAs are important for testing for compatibility between transplant donors and recipients (to find a good match and reduce the likelihood that the recipients antibodies will attack the organ), paternity testing (certain HLA patterns can be used to determine genetic relationships), and autoimmune disease diagnosis. Example: HLA-B27 is used for the diagnosis of spondyloarthropathies when clinical signs and symptoms are present. The identification of particular Human Leukocyte Antigens in association with autoimmune disease is not predictive. That is, people can have HLA-B27, and never go on to develop associated autoimmune diseases. But for those who do develop autoimmune disease, the presence of HLA-B27 can aid in diagnosis. HLAs are part of the complex collection of risk factors that, together with other factors, can lead to autoimmune disease. The study of Human Leukocyte Antigens and autoimmune disease has the potential to clarify and classify autoimmune disease.
Chromosome 6
HLAs are genetically determined by the DNA found in chromosome 6 (see the first image above). The DNA coding for HLAs is found at four distinct locations (loci) on chromosome 6.
Class I
Three DNA Locations: HLA-A, HLA-B, HLA-C
To be considered a Class I HLA means these antigens are expressed on all cells with a nucleus (the central part of a cell that contains a cell’s DNA), and platelets, in the human body. (Cruz-Tapias et. al, 2013)
Class II
Fourth Location: HLA-D
To be considered a Class II HLA means these types of antigens are expressed on Antigen Presenting Cells only. HLA-Ds are exclusively “expressed on antigen presenting cells (APC) such as B lymphocytes {a type of white blood cell}, dendritic cells {the body’s clean up crew}, macrophages {big eating cells}, monocytes, Langerhans cells, endothelial cells, and thymic epithelial cells.” (Cruz-Tapias et. al, 2013)
There are DNA Variations at These Locations
Variety is the spice of life—literally—as far as genetics is concerned. The HLA system contains the most genetic variety—differences between individuals—in the entire human genome (Cruz-Tapias et. al, 2013). The HLA system is part of the Major Histocompatibility (histo means tissue) Complex (MHC) and
The MHC has an essential role in the innate and adaptive immune system, and is characterized by high gene density, high polymorphism and high linkage disequilibrium. Much of what we know today about genetic variation and the organization of haplotypes was first discovered from studies of this region.
(Mungall et. al, 2003)
So, this area of chromosome 6 has lots of genes in one place, lots of variations in genes from person-to-person and “high linkage disequilibrium?” Gene variations (or alleles) “are in linkage disequilibrium (LD) when they do not occur randomly…Positive linkage disequilibrium exists when two alleles occur together…more often than expected” in “a large population with random mating.”
(Encyclopedia of Bioinformatics and Computational Biology, 2019)
(Brenner's Encyclopedia of Genetics (Second Edition), 2013)
HLA-DRB1, Polymyalgia rheumatica & Why It Matters
HLA-DRB1 is part of the Class II HLA-D location on chromosome 6. As a Class II HLA, HLA-DRB1 is expressed on antigen presenting cells only. Polymyalgia rheumatica was first associated with variations DRB1*0401 and *0404 (Haworth et. al, 1996). There is scant evidence to suggest that the variation HLA-DRB1*13:01 may be associated with the development of Polymyalgia rheumatica and Giant cell arteritis after influenza vaccination (Liozon et. al, 2021). More research and genetic analysis is needed to firmly establish the connection between HLA-DRB1 and Polymyalgia rheumatica. HLA-DRB1 variations have been studied in the context of other autoimmune diseases, particularly Rheumatoid arthritis, Multiple sclerosis, Systemic lupus erythematosus, Type 1 diabetes, and Sjogren’s syndrome. A class of autoimmune disease based on shared HLA-DRB1 variations has the potential to provide insight into shared disease mechanisms, shared treatments, and—I’ll just dream big here—early identification and prevention measures.
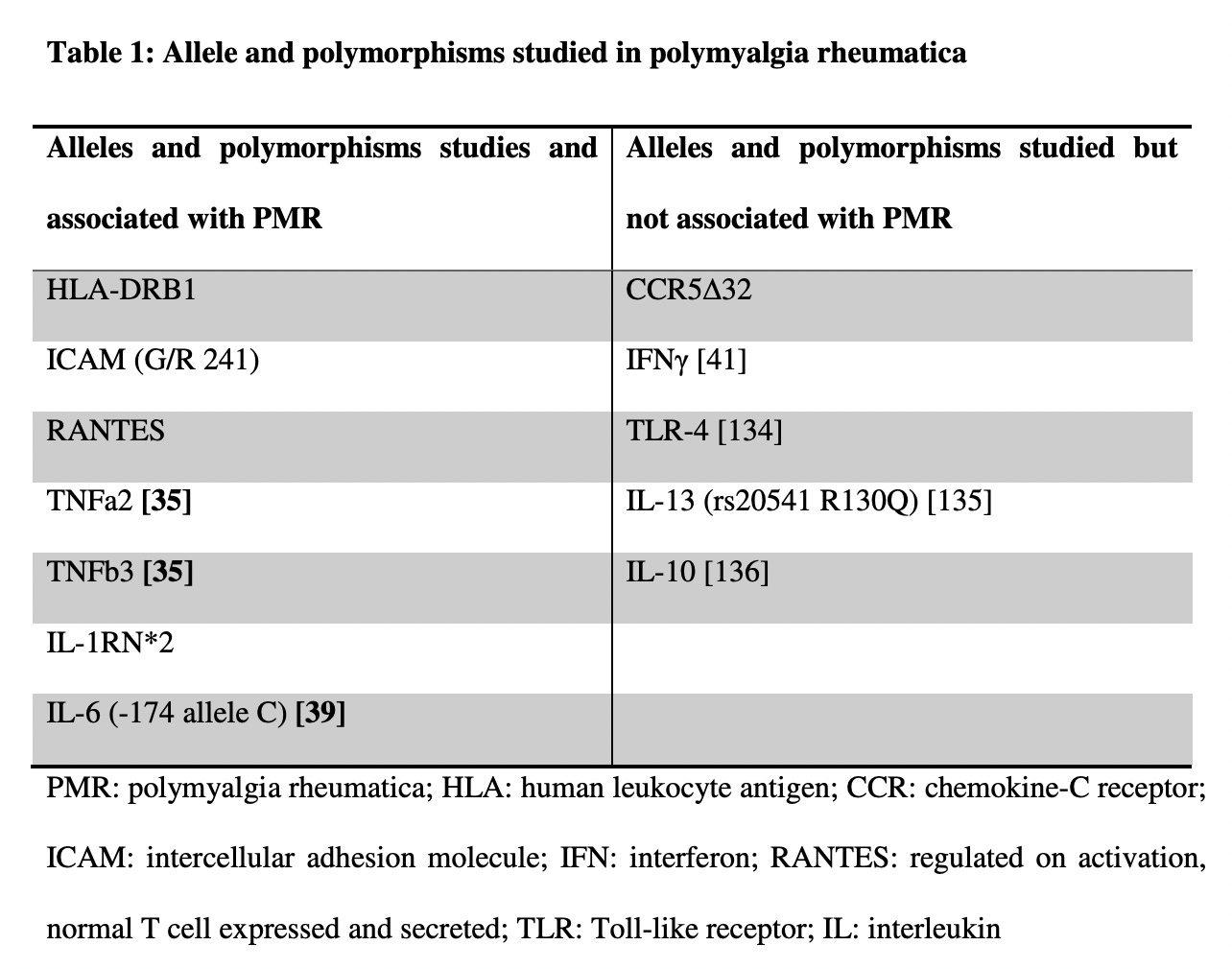
For reference, I’ve included a handy chart that summarizes studies that have associated Polymyalgia rheumatica with particular gene variations (most are not HLA associations), and also gene variations that are not associated with Polymyalgia rheumatica:
(Carvajal et. al, 2020)
This concludes my look at Polymyalgia rheumatica. Next week, I’ll be exploring Eosinophilic fasciitis. For those who are new to AutoimmuneDx, I am currently writing posts based on reader-requests for more information and analysis on particular autoimmune diagnoses. If you would like me to take a closer look at a particular diagnosis, please leave a comment below. If you don’t feel comfortable commenting publicly, email me at autoimmunedx@gmail.com. I will be on vacation next week, which will not interrupt next Friday’s post, but I will be slow to respond to comments or emails. I appreciate your patience, and your readership, so very much!
References
Carvajal Alegria G, Boukhlal S, Cornec D, Devauchelle-Pensec V. The pathophysiology of polymyalgia rheumatica, small pieces of a big puzzle. Autoimmun Rev. 2020 Nov;19(11):102670. doi: 10.1016/j.autrev.2020.102670. Epub 2020 Sep 15. PMID: 32942037.
Cruz-Tapias P, Castiblanco J, Anaya JM. Major histocompatibility complex: Antigen processing and presentation. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 10. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459467/
Haworth S, Ridgeway J, Stewart I, Dyer PA, Pepper L, Ollier W. Polymyalgia rheumatica is associated with both HLA-DRB1*0401 and DRB1*0404. Br J Rheumatol. 1996 Jul;35(7):632-5. doi: 10.1093/rheumatology/35.7.632. PMID: 8670595.
Liozon E, Parreau S, Filloux M, Dumonteil S, Gondran G, Bezanahary H, Ly KH, Fauchais AL. Giant cell arteritis or polymyalgia rheumatica after influenza vaccination: A study of 12 patients and a literature review. Autoimmun Rev. 2021 Feb;20(2):102732. doi: 10.1016/j.autrev.2020.102732. Epub 2020 Dec 14. PMID: 33326851.
Mungall, A., Palmer, S., Sims, S. et al. The DNA sequence and analysis of human chromosome 6. Nature 425, 805–811 (2003). https://doi.org/10.1038/nature02055